Researchers at MIT have developed an innovative technique to image nanoscale structures in cells without relying on expensive, high-powered super-resolution microscopes. This method involves expanding tissues before imaging, allowing conventional optical microscopes to achieve nanoscale resolution.
In the latest advancement of this technique, researchers have managed to expand tissues by 20 times in a single step. This straightforward and cost-effective method could enable nearly any biology lab to perform nanoscale imaging.
"This democratizes imaging," says Laura Kiessling, a chemistry professor at MIT and a member of both the Broad Institute of MIT and Harvard and MIT’s Koch Institute for Integrative Cancer Research. "Without this method, achieving high-resolution imaging requires very expensive microscopes. This new technique allows you to see things that are normally invisible with standard microscopes, reducing imaging costs by eliminating the need for specialized facilities."
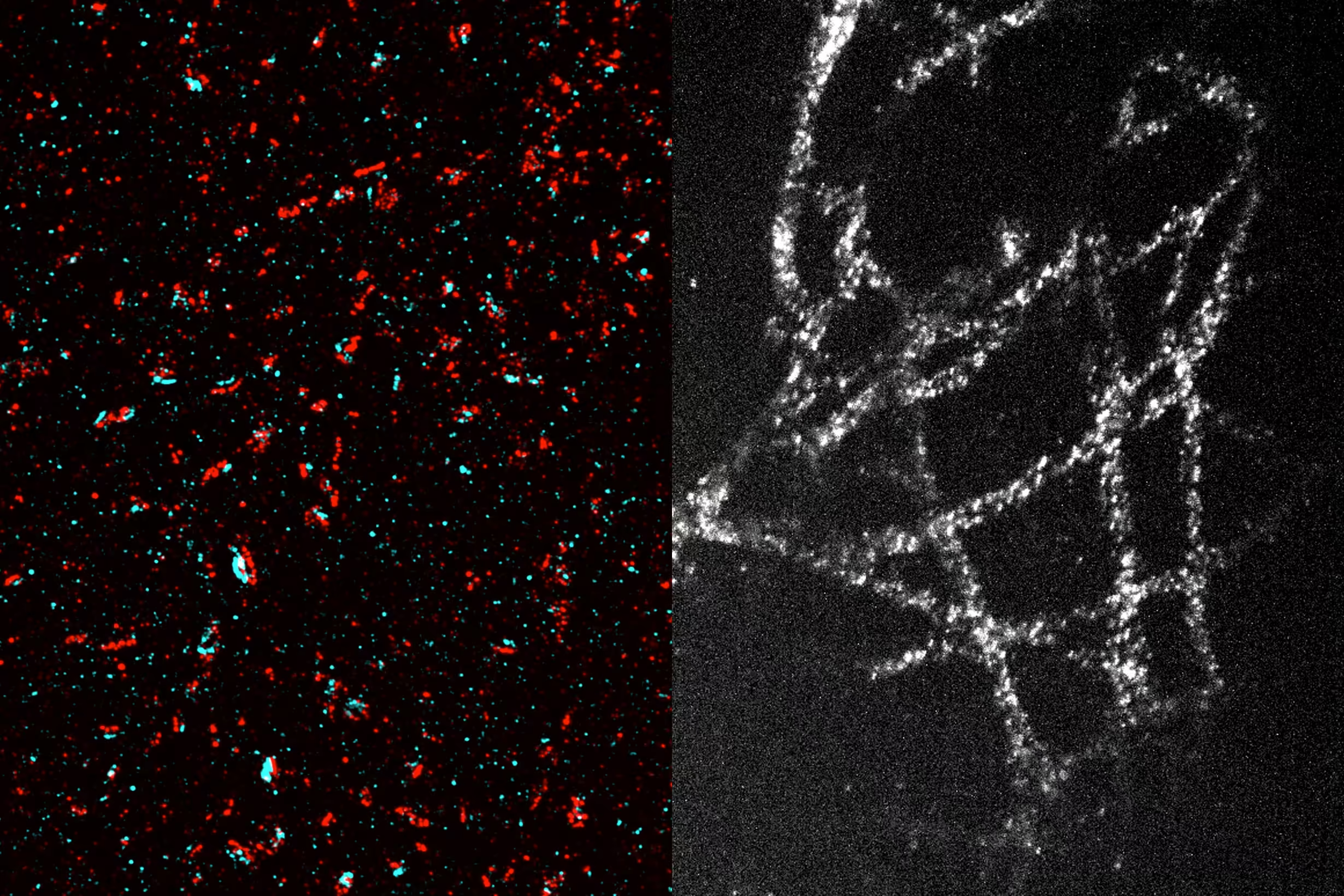
With this technique, which achieves a resolution of about 20 nanometers, scientists can observe organelles inside cells and protein clusters.
"A 20-fold expansion brings you into the realm where biological molecules operate. The building blocks of life are nanoscale: biomolecules, genes, and genetic products," explains Edward Boyden, a professor at MIT and a Howard Hughes Medical Institute investigator.
Boyden and Kiessling are the senior authors of the new study, published today in Nature Methods. The lead authors are Shiwei Wang, a graduate student at MIT, and Tay Won Shin, PhD ’23.
Single-Step Expansion
Boyden’s lab introduced expansion microscopy in 2015. This technique involves embedding tissues in an absorbent polymer and breaking the proteins that normally hold tissues together. Adding water causes the gel to swell, separating biomolecules.
The original version expanded tissues fourfold, achieving a resolution of about 70 nanometers. In 2017, Boyden’s lab introduced a second expansion step, achieving a 20-fold expansion for even higher resolution, though the process was more complex.
"We’ve developed several 20-fold expansion technologies in the past, but they required multiple expansion steps," Boyden says. "Achieving such expansion in a single step simplifies the process."
With a 20-fold expansion, researchers can achieve approximately 20-nanometer resolution using a conventional optical microscope, allowing them to see cellular structures like microtubules and mitochondria, as well as protein clusters.
In the new study, researchers aimed for a 20-fold volume expansion in one step. This required finding a gel that was both highly absorbent and mechanically stable to prevent disintegration during expansion.
They used a gel made from N,N-dimethylacrylamide (DMAA) and sodium acrylate. Unlike previous expansion gels that relied on adding another molecule to form cross-links between polymer strands, this gel spontaneously forms cross-links and has strong mechanical properties. Although similar gel components had been used in previous expansion microscopy protocols, they only expanded about tenfold. The MIT team optimized the gel and polymerization process to make the gel more robust and enable 20-fold expansion.
To further stabilize the gel and improve reproducibility, researchers removed oxygen from the polymer solution before gelation, preventing side reactions that interfere with cross-linking. This step involves passing nitrogen gas through the polymer solution to replace most of the oxygen.
Once the gel forms, certain protein bonds holding tissues together are broken, and water is added to expand the gel. After expansion, target proteins in the tissues can be labeled and imaged.
"This approach may require more sample preparation compared to other super-resolution techniques, but it’s much simpler for the imaging process itself, especially for 3D imaging," Shin explains. "We document the step-by-step protocol in the manuscript so readers can easily follow it."
Imaging Tiny Structures
Using this technique, researchers have visualized numerous tiny structures within brain cells, including synaptic nanocolumns. These are clusters of proteins arranged specifically at neuronal synapses, allowing neurons to communicate via neurotransmitters like dopamine.
In cancer cell studies, researchers imaged microtubules, hollow tubes that help maintain cell structure and play a crucial role in cell division. They also observed mitochondria (energy-generating organelles) and even the organization of individual nuclear pore complexes (protein clusters controlling access to the cell nucleus).
Wang is now using this technique to image glycans, carbohydrates on cell surfaces that help control cell interactions with their environment. This method could also be used to image tumor cells, providing insights into protein organization within these cells more easily than before.
Researchers envision that any biology lab could use this low-cost technique, as it relies on standard, commercially available chemicals and common equipment like confocal microscopes and glove boxes, which most labs already have or can easily access.
"We hope that with this new technology, any conventional biology lab can use this protocol with their existing microscopes, allowing them to achieve a resolution only attainable with highly specialized and expensive advanced microscopes," Wang says.
The research was funded in part by the U.S. National Institutes of Health, a MIT Presidential Graduate Fellowship, U.S. National Science Foundation Graduate Research Fellowships, Open Philanthropy, Good Ventures, the Howard Hughes Medical Institute, Lisa Yang, Ashar Aziz, and the European Research Council.